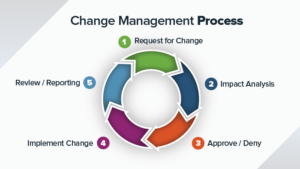
CAPA process for regulatory compliance
The activities related to Continual Improvement provides foundation block for growth and sustainability of the organizations. Corrective and Preventative actions (CAPA) system is one of the many CI approach that is intuitive and easy to Implement. The CAPA system is also required by the ISO9000 standards, 21CFR part 820 for medical devices. To comply with the above standards and regulations, we would need to implement a closed loop CAPA system that is effective, auditable and traceable.