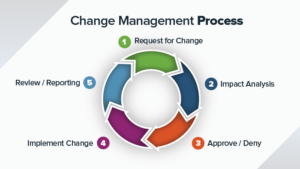
The following section describes a sample SOP (standard operating procedure) for corrective and preventive actions (CAPA) in your Quality Management System (QMS). Please contact us...
Simple Guide for ISO13485 Auditing
This document provides simple guidance and details you would need to set up your internal auditing process to comply with the ISO13485 standard and FDA...