If you are running a calibration management operation with paper based or Excel spreadsheets, it may be taking lot of your valuable time scheduling, tracking...
Document Change Control for QMS
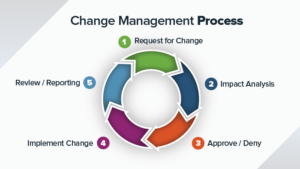
The Document Change Control is one of the key requirements for ISO9001, ISO13485 and other ISO standards. Effective Document Change Control is one of the...
Employee Training Program
Why do we need to train employees? Why do we need to keep training records for employee training program?Managing the employee training program is one...