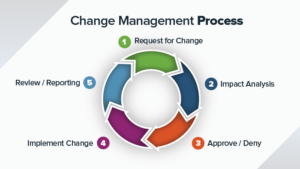
It is challenging to manually manage Corrective and Preventative Action (CAPA) process with paper based or Excel file system. Here are some of the key...
News
What is Design Control of Medical Devices
The Design Control of Medical Devices includes but not limited to creation, change and reviews of design data, including but not limited to, design input...
Medical Device Quality Risk Management
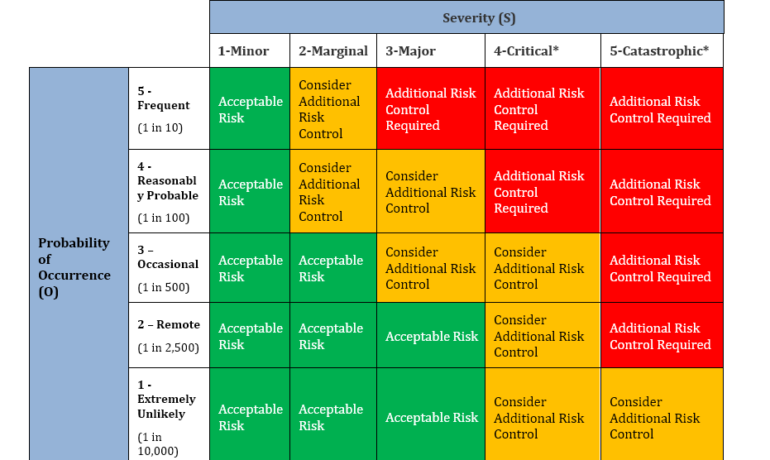
https://www.slideshare.net/SanjayDhal/risk-management-in-qms-and-new-products-design-npd By adding quality risk management into your processes, especially at the design and planning phase, you can take actions to ensure that anticipated...
QMS Software for ISO13485
There are many challenges involved when implementing a QMS system that will comply with ISO13485:2016. Here are top 10 requirements you should focus on early...