The Document Change Control is one of the key requirements for ISO9001, ISO13485 and other ISO standards. Effective Document Change Control is one of the mandatory requirement s for compliance with 21 CFR 820.
PART 820 — QUALITY SYSTEM REGULATION
Subpart D–Document Controls
| Sec. 820.40 Document controls. |
| Each manufacturer shall establish and maintain procedures to control all documents that are required by this part. The procedures shall provide for the following: (a) Document approval and distribution. Each manufacturer shall designate an individual(s) to review for adequacy and approve prior to issuance all documents established to meet the requirements of this part. The approval, including the date and signature of the individual(s) approving the document, shall be documented. Documents established to meet the requirements of this part shall be available at all locations for which they are designated, used, or otherwise necessary, and all obsolete documents shall be promptly removed from all points of use or otherwise prevented from unintended use. (b) Document changes. Changes to documents shall be reviewed and approved by an individual(s) in the same function or organization that performed the original review and approval, unless specifically designated otherwise. Approved changes shall be communicated to the appropriate personnel in a timely manner. Each manufacturer shall maintain records of changes to documents. Change records shall include a description of the change, identification of the affected documents, the signature of the approving individual(s), the approval date, and when the change becomes effective. |
Document Change Control System is intended to control all documents related to QMS(Quality Management System) and Manufacturing Operations compliance of the facility and procedures performed therein. Controlled documentation refers to any documentation used in the manufacturing and Quality Systems operations performed at a Company (e.g. Qualcy Systems Inc – QSI). It shall be used to provide integrity and consistency to methodologies used in the QSI operations. This document provides guidance to establish a document change control procedure using Qualcy eQMS software.
Who is Responsible?

Document Change Control Management
Documents will be created and revised by the party or parties with primary responsibility for the areas concerned. For example, the QMS operating documents shall be created and updated by the Quality Manager or the designee, the facility protocols and procedures will be created by the staff of QSI; study protocols and instrument IQ/OQ/PQ documentation will be produced by the validation contractor and may be supplied, in part, by the equipment manufacturer.
The Quality Assurance Manager or the designee will be responsible for controlling electronic and printed documents.
It is the responsibility of all QSI employees to be aware of and use the Approved and Released revision and version of controlled documentation. The Approved and Released revision of the controlled documents are available in the Qualcy EQMS virtual Released Cabinet location.
Process involved in Document Change Control
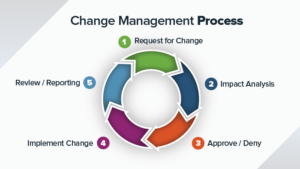
Document Change Control Management
Document Change Control Initiation
(1) To create a new document, the Quality Manager or the designee shall be contacted to create the document number and the name of the document in the Table of Contents. Then the Quality Manager or the designee will create a new DocFolder with new number and the description in the Qualcy EQMS system. The Quality Manager or the designee will assign the Approval matrix in the DocFolder. The newly created DocFolder resides in the virtual Draft Cabinet.
(2) After the creation of the DocFolder, the employees can create and upload the new content documents into the DocFolder. All initial content documents should be created using the approved and appropriate templates. The employees shall fill in additional information in the DocFolder as needed.
(3) To release a new DocFolder, a new content change request (CCR) is to be created and routed for approval in the Qualcy EQMS system. The DocFolder created in the previous step has to be attached (or checked in) to the CCR. Then the CCR has to be submitted for review. The CCR is automatically routed to the approvers for review. Once the approvers complete their approval, the attached DocFolders get released to the Virtual Released Cabinet. At this point, the status of the DocFolder shall have changed to Approved and Released. The status of CCR shall have been changed to the Approved and Released.
Document Revision

Document Change Control Management
(1) To revise an existing DocFolder in the Qualcy EQMS system, the initiator must check out a copy of the DocFolder from the Released Cabinet to Draft Cabinet. The revision number and version number of the DocFolder are automatically updated by the system. The initiator shall make the changes to the content document inside the DocFolder. Then the initiator has to create a new CCR with CCR type of ToRelease. Then the initiator will upload the DocFolder from the previous step into the new CCR. Then the initiator will submit the CCR for review by the approvers. After the approval of the CCR, the revised DocFolder gets released to Released Cabinet. At this point, the status of the DocFolder shall have changed to Approved and Released. The status of CCR shall have been changed to the Approved and Released.
(2) When creating a new CCR all the fields marked with red asterisks (*) must be filled in. When submitting a new CCR for review, all the fields marked with black asterisks (*) must be filled in.
(3) The existing revision numbers of the legacy documents will be discontinued and new revision number starting with Rev1 will be assigned in the Qualcy EQMS system. The above change will be permitted following the approval and release of this document.
Number/Revision Assignment
(1) New DocFolders will be assigned the next available number using the format mentioned in section 4.9. The table of contents shall be updated accordingly.The revision number and version number of the DocFolders will be automatically assigned and updated by the Qualcy EQMS system.
Document Review and Approval
Document Change Control Management
(1) The Quality Assurance Manager or the designee and the General Manager will review the CCRs in a timely manner. DocFolder Upon review the approver (s) will :
(2) Reject the CCR and submitted document. The approver should provide reason for rejection in the comments field on the workflow page; at this point the CCR is routed back to the initiator. The status of the CCR shall have been changed to the previous status.
(3) Approve the CCR and submitted document (reviewing for accuracy, completeness, and correctness).
(4) Once the CCR is approved, the revised DocFolder gets released to Released Cabinet. At this point, the status of the DocFolder shall have changed to Approved and Released. The status of CCR shall have been changed to the Approved and Released. The previous revision of the DocFolder gets automatically moved to the virtual Archived Cabinet.
You can contact us to set up a demo to see the Qualcy Document Management Software. Also you can get a word copy of this document.