Ever stared at a mountain of QMS documentation wondering if there’s a better way? For medical device companies, quality management isn’t just paperwork—it’s patient safety on the line.
The reality? Most quality teams spend 60% of their time on manual documentation rather than actual quality improvement. That’s where AI in quality management systems is changing everything. Problem solving and data analysis is done for you vs. you spending time to do it.
Think about this: what if your QMS could predict compliance issues before they happen? What if it could auto-generate SOPs that actually make sense? The smartest medical device manufacturers aren’t just implementing AI—they’re using it to transform how they approach quality altogether. The AI is helping companies see the connection between the processes and implement actions proactively.
- Understanding QMS Challenges in Medical Device Companies
Regulatory complexity in medical device quality management
Medical device companies are drowning in regulations. It’s not just FDA or EU MDR—it’s a global maze of constantly changing requirements. One day you’re compliant, the next you’re scrambling because Japan updated their standards.
The real headache? These regulations don’t just stack—they overlap and sometimes contradict each other. You might satisfy FDA requirements only to discover your EU documentation falls short.
Most quality teams are stuck using spreadsheets and email chains to track these requirements. That’s like using a paper map to navigate through rush hour traffic when everyone else has GPS.
Documentation burden and compliance costs
The paperwork is ridiculous. For a single medical device, you’re looking at thousands of documents—design history files, risk assessments, clinical data, manufacturing records…the list never ends.
And maintaining these documents? It’s a full-time job for entire teams. Every tiny product change triggers a documentation avalanche. A simple component update might require revising 50+ documents.
The financial hit is massive:
-
- Quality staff salaries (often 15-20% of operational costs)
- External consultants ($200-500/hour)
- Training ($1,500-3,000 per employee annually)
- Systems maintenance ($100K+ annually for enterprise solutions)
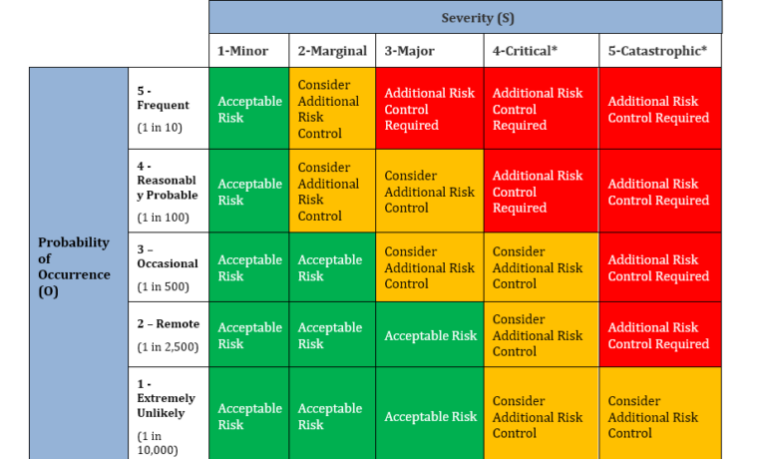
Risk management requirements specific to medical devices
Risk management for medical devices isn’t like other industries. When your product goes into someone’s body, “acceptable risk” takes on a whole new meaning.
ISO 14971 demands you track risks throughout the entire product lifecycle. That means constantly monitoring:
-
- Design risks
- Manufacturing risks
- User errors
- Long-term performance issues
The trickiest part? Balancing risk mitigation with usability. Make a device too complicated in the name of safety, and you create new risks through user confusion.
Audit preparation and inspection readiness
Nothing strikes fear quite like hearing “FDA inspection next month.” Suddenly, everyone’s working weekends, digging through files, and praying nothing’s missing.
Most companies’ audit prep looks like this:
-
- Panic
- Rush to gather evidence
- Discover missing documents
- Create documents retrospectively (yikes!)
- Hope auditors don’t notice
- AI Capabilities Transforming Quality Management
Automated document control and version management
Gone are the days of chasing paper trails and manually tracking document versions. AI transforms this tedious work into a smooth, error-free process. Modern AI tools can:
-
- Automatically route documents for review based on content type
- Flag inconsistencies between related documents
- Track document access and modifications in real-time
- Predict when documents need review based on regulatory timelines
One medical device manufacturer reduced document processing time by 67% after implementing AI document control. The system even caught critical conflicts between their design specifications and risk assessment documentation that humans had missed for months.
Intelligent deviation and CAPA processing
AI doesn’t just spot problems – it helps solve them faster. When deviations occur, AI systems can:
-
- Categorize issues based on historical patterns
- Recommend appropriate corrective actions
- Identify root causes by analyzing manufacturing data
- Predict potential effectiveness of proposed solutions
A Class II device maker told us they slashed CAPA closure times from 45 days to just 11 days using AI assistance. Their system now flags repeat issues across different manufacturing lines that previously went unnoticed as connected problems.
Predictive quality analytics and trend identification
Why wait for failures when AI can see them coming? Advanced algorithms now:
-
- Detect subtle patterns in production data before issues emerge
- Identify correlations between seemingly unrelated quality factors
- Project future nonconformance rates based on current conditions
- Recommend preventive actions prioritized by risk level
These capabilities translate to real savings. One cardiovascular device company identified a critical component degradation issue three months before it would have affected patients by using predictive analytics on their post-market surveillance data.
Natural language processing for regulatory updates
Keeping up with regulatory changes is a nightmare. NLP systems make it manageable by:
-
- Scanning global regulatory databases for relevant updates
- Highlighting specific sections impacting your product categories
- Translating complex regulatory language into actionable tasks
- Automatically updating quality documentation with new requirements
One of our customers integrated NLP into their QMS and reduced regulatory research time by 82%. Their system flagged a minor EU MDR update that competitors completely missed.
Machine learning for risk assessment
The old risk matrices and FMEA tables are getting a serious upgrade. ML-powered risk assessment:
-
- Evaluates potential failure modes based on historical data
- Provides more accurate probability estimates than human guesses
- Recommends risk controls prioritized by effectiveness
- Continuously refines risk models as new data becomes available
The results speak for themselves. A diagnostic equipment manufacturer applied ML to their risk assessment process and identified 23% more potential failure modes than their traditional approach, while cutting assessment time in half.
The reactive scramble costs thousands in overtime and emergency consulting—all while normal operations grind to a halt. And that’s assuming you pass. The alternative? Warning letters, recalls, or even facility shutdowns.
- Implementation Strategies for AI-Powered QMS
Assessing QMS processes suitable for AI enhancement
Not every QMS process needs AI—but some are begging for it. Start by looking at your data-heavy processes. Document control systems drowning in paperwork? CAPA management taking forever? Those are prime AI candidates.
The best targets have these traits:
-
- Repetitive tasks eating up staff time
- Processes with clear patterns and rules
- Areas where you’re constantly playing catch-up
- Functions requiring quick analysis of tons of data
Take complaint handling. AI can scan incoming complaints, categorize them, spot trends, and flag high-risk issues before your morning coffee gets cold. Meanwhile, your human experts can focus on solving complex problems instead of sorting emails.
Data integration and system architecture considerations
Your AI system is only as good as the data feeding it. Garbage in, garbage out—but worse, because now it’s automated garbage.
A solid foundation needs:
-
- Clean, consistent data sources
- Standardized formats across systems
- APIs that actually talk to each other
- Cloud infrastructure that scales with your needs
Don’t build a Ferrari on dirt roads. If your quality data lives in five different systems plus seventeen spreadsheets on someone’s desktop, fix that first. The best AI implementations start with a unified data strategy.
And remember security—medical device quality data is sensitive stuff. Your architecture should include encryption, access controls, and audit trails that would make a hacker cry.
Validation approaches for AI tools in regulated environments
The FDA doesn’t care how cool your AI is if you can’t prove it works reliably. Validating AI tools in medical device QMS requires a different approach than traditional software.
You’ll need to:
-
- Document your training data sources and cleaning methods
- Set performance metrics relevant to quality outcomes
- Establish baselines for comparing AI vs. human performance
- Create test cases for edge scenarios that might break the system
Build validation directly into your development process. Your documentation should explain not just what the AI does but why it makes specific decisions. “It’s a black box” won’t fly with auditors.
Change management and staff training
People resist change. They resist it even more when that change sounds like it might replace them.
The human side of AI implementation requires:
-
- Clear communication about what AI will (and won’t) do
- Early involvement of quality teams in tool selection
- Hands-on training with real-world scenarios
- Designated “AI champions” who help peers adapt
Don’t drop an AI system on your team without warning. Show them how it handles the boring stuff while making their expertise more valuable. The most successful implementations pair AI capabilities with human judgment—neither works well alone.
Create feedback loops where staff can report issues and see improvements. When people feel ownership of the AI tools, adoption rates skyrocket.
- Real-World Applications of AI in Medical Device QMS
Automated supplier qualification and monitoring
Ever spent weeks evaluating potential suppliers? AI is changing that game completely. Smart algorithms now scan thousands of supplier records in minutes, flagging risks that humans might miss. One medical device manufacturer cut their supplier qualification time by 73% using AI that automatically verified certifications and analyzed quality history.
The beauty is in the continuous monitoring. AI doesn’t just qualify suppliers once and forget them. It watches performance metrics in real-time, sending alerts before small issues become FDA violations.
Smart complaint handling and post-market surveillance
Gone are the days of manually sorting through customer complaints. AI systems now categorize incoming issues instantly, prioritize based on risk profiles, and even suggest corrective actions.
AI tools dig through social media, clinical literature, and adverse event databases to spot potential problems before they escalate. One cardiac device company caught a potential issue three months earlier than they would have through traditional surveillance methods.
Design control and product development workflow optimization
AI doesn’t just streamline paperwork—it transforms how medical devices are designed.
Design verification? AI predicts test results before you run them. Risk management? Algorithms identify hazards humans might overlook. Design reviews? The system flags inconsistencies across documents automatically.
The time savings are dramatic. Companies report cutting development cycles by 30-40% while actually improving compliance.
Manufacturing quality control and real-time monitoring
The factory floor is where AI really shines. Computer vision systems inspect devices with precision humans can’t match. Predictive maintenance algorithms spot equipment issues before failures occur.
What’s revolutionary is the continuous learning. These systems get smarter with every production run, reducing defects and false rejections simultaneously.
Regulatory submission preparation and management
Preparing submissions for FDA or EU MDR approval used to mean months of document preparation. AI now extracts relevant data from your QMS, formats it to regulatory specifications, and flags missing elements.
The system even suggests appropriate predicate devices and helps predict regulatory questions. One company reduced submission preparation time from 8 months to 10 weeks while improving first-time approval rates.
- Measuring ROI and Performance Improvements
Key performance indicators for AI-enhanced QMS
Tracking the right KPIs is crucial when implementing AI in your medical device QMS. The numbers don’t lie – and they’ll tell you exactly how your investment is paying off.
Most successful medical device companies focus on these primary KPIs:
-
- Processing time reduction: How much faster are your document approvals and change controls moving?
- Error detection rate: Are you catching more issues before they become problems?
- Prediction accuracy: How well is your AI forecasting potential compliance risks?
- User adoption rate: Are your teams actually using the AI tools available to them?
Compliance efficiency metrics
The FDA doesn’t care about your fancy AI – they care if you’re compliant. But your AI should make compliance dramatically easier.
Track these metrics to prove it:
-
-
- Audit preparation time: Companies using AI typically cut prep time by 40-60%
- Time to resolution for findings: AI-assisted systems resolve audit findings 3x faster
- Regulatory submission cycles: Each rejection costs months and millions – AI reduces rejections by identifying gaps earlier
-
Cost reduction analysis
Money talks. When leadership asks about the ROI, have these numbers ready:
| Cost Category | Typical AI Impact |
| Documentation | 65% reduction in processing costs |
| Training | 40% decrease in compliance training expenses |
| Quality events | 50% reduction in investigation costs |
| Audit costs | 35-55% lower external consultant fees |
Quality improvement outcomes
The ultimate measure isn’t just saving money – it’s making better, safer products.
Real impact shows up in:
-
- Decreased complaint rates: AI pattern recognition identifies root causes faster
- Reduced CAPA cycle times: From 90+ days down to 30-45 days on average
- Increased first-pass yield: Better predictive maintenance means fewer defects
- Faster market response: When issues arise, AI helps you implement corrections 2-3x faster
Smart companies don’t just implement AI – they measure its impact rigorously. The numbers will speak for themselves.
- Future Trends in AI-Driven Quality Management
Regulatory Authority Perspectives on AI in Quality Systems
The FDA and EMA are warming up to AI in medical device quality systems—but they’re not jumping in blindly. They want the good stuff (efficiency, better compliance) without the scary parts (black box algorithms making critical decisions).
What’s actually happening right now? The FDA’s Digital Health Center of Excellence is building frameworks for AI validation, while the EU’s MDR includes specific provisions for AI-enabled medical technologies.
Regulatory authorities expect:
-
- Transparent algorithms with audit trails
- Validation protocols specific to AI systems
- Human oversight (they’re not letting robots run the show yet)
The FDA’s “Artificial Intelligence and Machine Learning in Software as a Medical Device” guidance shows they’re getting serious about standardizing expectations. But there’s still that regulatory tightrope—wanting innovation without compromising safety.
Advanced Predictive Capabilities for Proactive Compliance
AI is shifting quality management from “fix it when it breaks” to “prevent it from breaking.” Smart.
The latest predictive systems can:
-
- Spot patterns in thousands of CAPA records to identify underlying systemic issues
- Predict potential audit findings before inspectors walk through the door
- Flag documentation inconsistencies that humans miss
These systems learn from historical compliance data across multiple facilities and even different companies (anonymized, of course). One medical device manufacturer reduced non-conformances by 47% after implementing AI prediction models that analyzed production line variables alongside quality metrics.
Integration with Digital Twin Technology
Digital twins are changing the game for medical device companies. These virtual replicas simulate real-world conditions to test quality parameters without risking actual products.
By creating digital twins of:
-
- Manufacturing processes
- Supply chains
- Even entire quality systems
Companies can run “what-if” scenarios to optimize procedures before implementation. When integrated with AI, these twins become even more powerful—continuously learning from real-world performance data.
The practical payoff? A Class III device manufacturer used digital twin technology to reduce validation cycles by 35% while improving first-time validation success rates.
Blockchain for Secure Quality Records
Blockchain isn’t just for crypto. It’s revolutionizing quality record integrity for medical devices.
The immutable nature of blockchain creates tamper-proof documentation chains that:
-
- Ensure complete traceability throughout product lifecycles
- Create verifiable audit trails that satisfy even the pickiest regulators
- Enable secure sharing of quality data with regulatory bodies
Some forward-thinking companies are already implementing blockchain to track components from suppliers through manufacturing and into distribution—creating unbreakable chains of custody.
This technology addresses one of the biggest headaches in quality management: proving your records haven’t been altered after the fact. When the FDA questions your documentation, having blockchain-verified records provides rock-solid evidence of compliance
Conclusion
Revolutionizing Quality Management
AI integration offers medical device companies powerful solutions to their most pressing QMS challenges. From automating documentation and streamlining compliance to enhancing risk management and predictive maintenance, these technologies deliver measurable improvements in efficiency, accuracy, and regulatory alignment. The implementation strategies outlined provide practical pathways for companies at various stages of technological maturity to incorporate AI into their quality management processes.
As the regulatory landscape continues to evolve, AI-powered QMS systems will become increasingly essential for medical device manufacturers. Companies that embrace these technologies now will not only achieve immediate operational benefits and ROI but will also position themselves advantageously for future innovations in regulatory intelligence and adaptive quality management. The time to explore AI implementation in your QMS is now—your compliance efforts, product quality, and bottom line will thank you.